
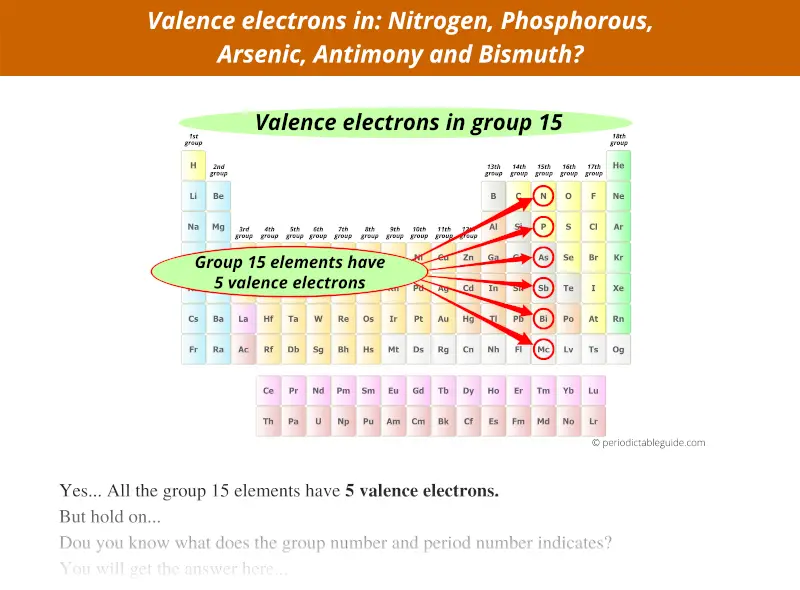
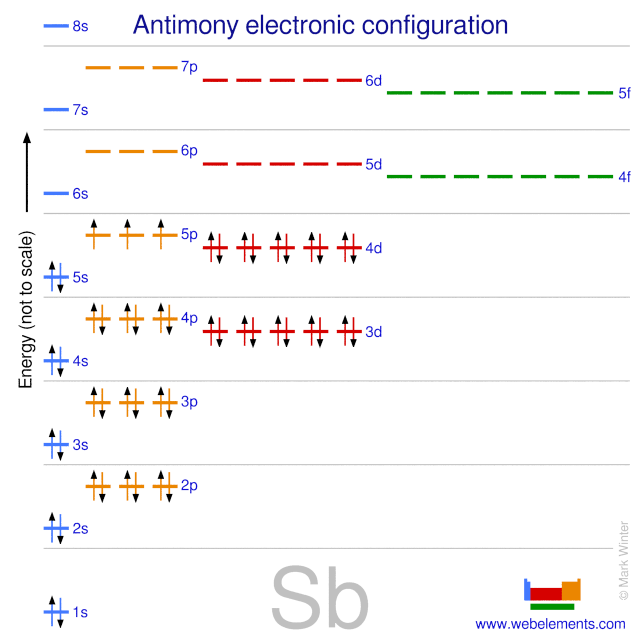
What is the melting Point of Antimony in Kelvin? What is the boiling Point of Antimony?īoiling Point of Antimony is 1860 K. Antimony has 51 electrons out of which 5 valence electrons are present in the 5s2 5p3 outer orbitals of atom. How many valence electrons does a Antimony atom have?Īntimony has 5 valence electrons. The element Antimony was discovered by Arabic alchemist in year ca. What is the color of Antimony?Īntimony is of Silver color. It is located in group 15 and period 5 in the modern periodic table. Antimony is the 51 element on the periodic table. What is the position of Antimony in the Periodic Table?Īntimony is a chemical element with the symbol Sb and atomic number 51. Antimony is a chemical element with symbol Sb and atomic number 51. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. The abbreviated electronic configuration of Antimony is 4d10 5s2 5p3. What is the abbreviated electronic configuration of Antimony? The electronic configuration of Antimony is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p3. What is the electronic configuration of Antimony? Optical Properties of Antimony Refractive IndexĪcoustic Properties of Antimony Speed of SoundĪntimony Thermal Properties - Enthalpies and thermodynamics

Refer to table below for the Electrical properties ofAntimony Electrical ConductivityĪntimony Heat and Conduction Properties Thermal ConductivityĪntimony Magnetic Properties Magnetic Type Hardness of Antimony - Tests to Measure of Hardness of Element Mohs HardnessĪntimony is Conductor of electricity. Refer to below table for Antimony Physical Properties DensityĦ.697 g/cm3(when liquid at m.p density is $6.53 g/cm3)


 0 kommentar(er)
0 kommentar(er)
